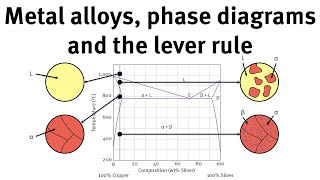
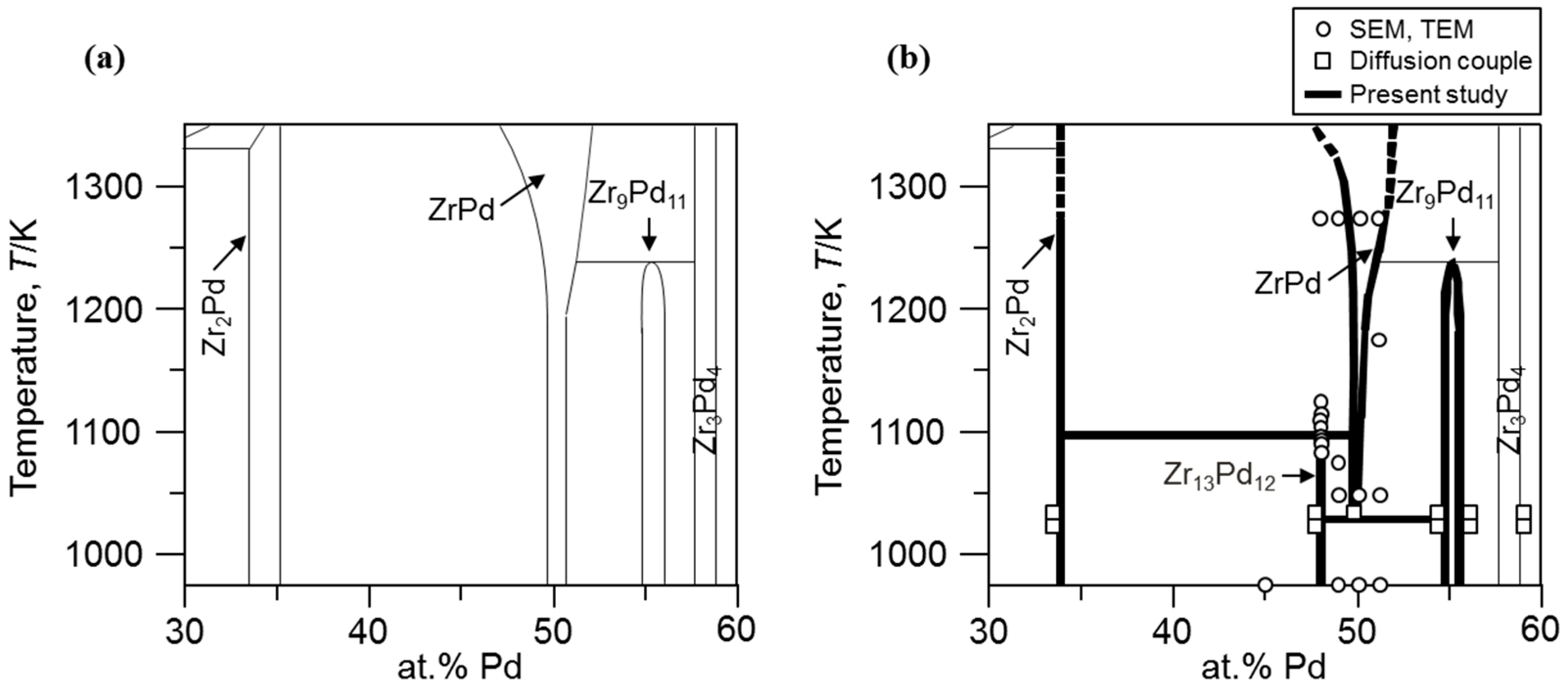
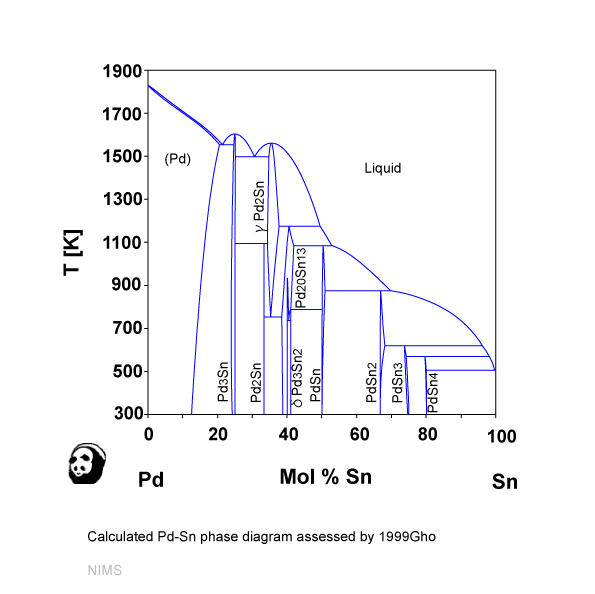
The calculated Pb-Pd phase diagram (a) with experimental points (Ref 3,... | Download Scientific Diagram

A lead-tin mixture containing 40 wt% tin is cooled from about 330^oC to 182^oC. These points are shown as points A and B. Calculate the amount of eutectic \alpha present in the

The Characterization of the Stannous Chloride/ Palladium Chloride Catalysts for Electroless Plating | Products Finishing
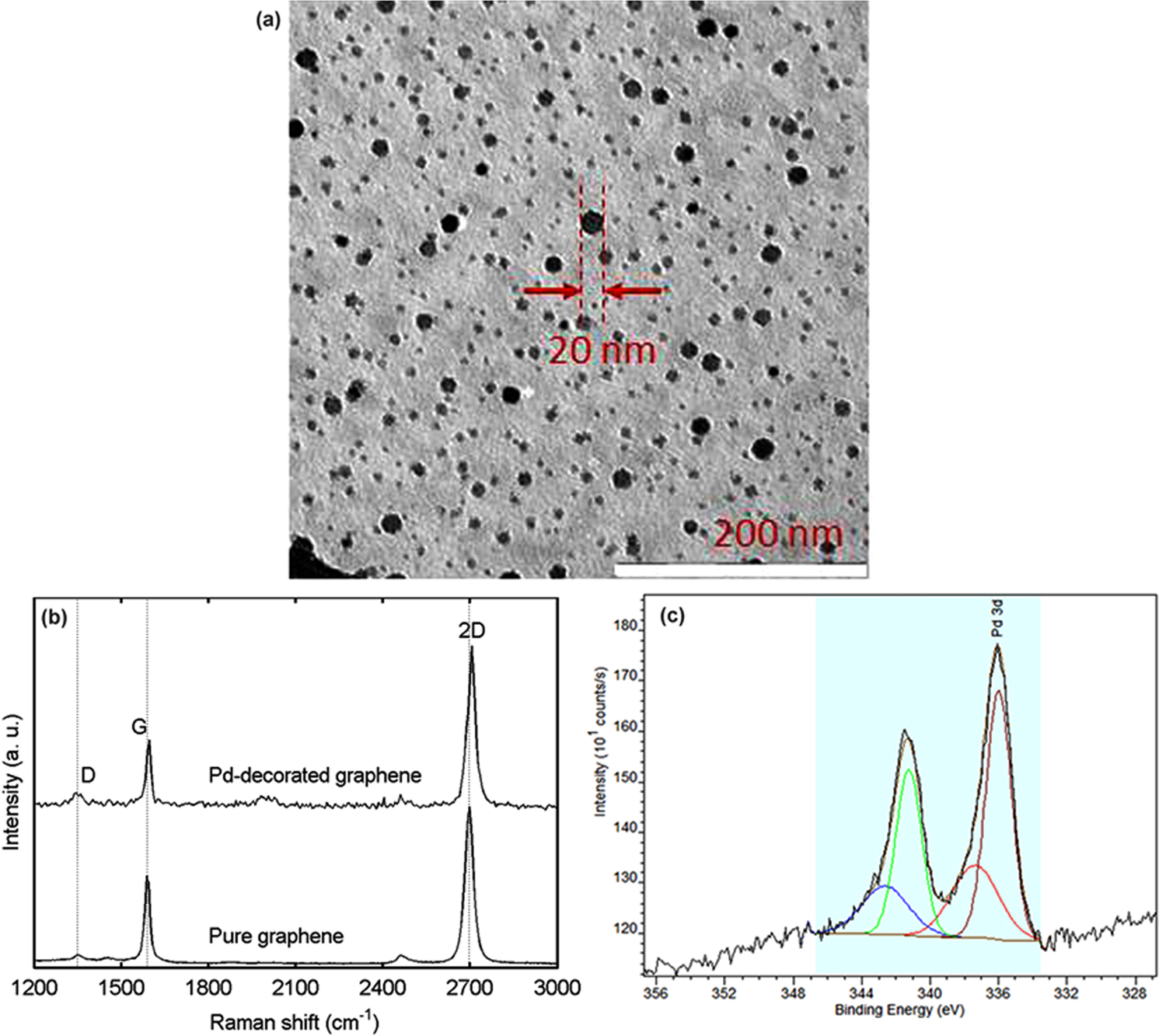
Chemically deposited palladium nanoparticles on graphene for hydrogen sensor applications | Scientific Reports

Phase Control of Solid-Solution Nanoparticles beyond the Phase Diagram for Enhanced Catalytic Properties | ACS Materials Au

Vapor phase deoxygenation of heptanoic acid over silica-supported palladium and palladium-tin catalysts - ScienceDirect

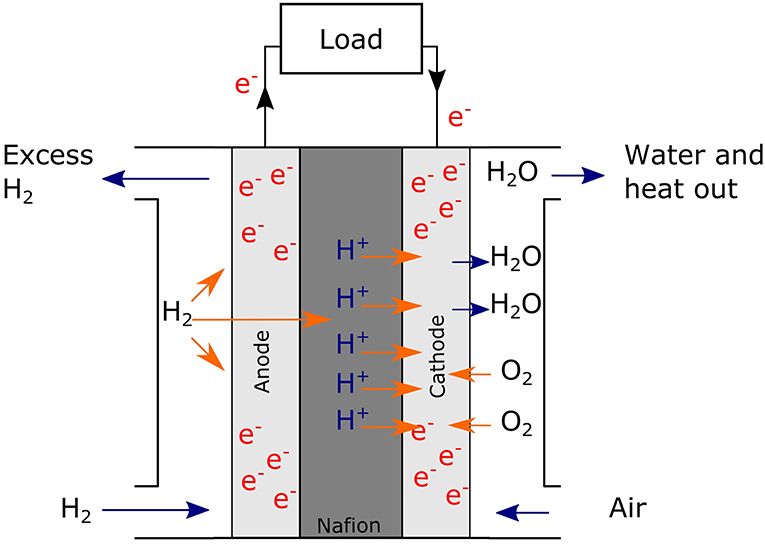
Recent progress in palladium-nonmetal nanostructure development for fuel cell applications | NPG Asia Materials

Alkaline Formate Oxidation with Colloidal Palladium–Tin Alloy Nanocrystals | ACS Applied Energy Materials


![Phase diagram of the palladium-platinum binary metal system [1] | Download Scientific Diagram Phase diagram of the palladium-platinum binary metal system [1] | Download Scientific Diagram](https://www.researchgate.net/publication/348604433/figure/fig1/AS:1022471264272384@1620787554327/Phase-diagram-of-the-palladium-platinum-binary-metal-system-1.png)